- - animals:
- - type:
- anti parasites
- - usage:
- Oral Solution
- - manufacturer:
- Rooyan Darou
- - Packaging:
- 0.5, 1 and 4 L polyethylene containers.
Product properties
Composition
Each ml the product contains 1 mg Moxidectin& 50 mg of Triclabendazole.
Pharmacology:
Moxidectin is an endectocide active against a wide range of internal and external parasites and is a second generation of macrocyclic lactone of the milbemycin family. Its principal mode of action is interfering with neuromuscular transmission of the GABA (gamma amino butyric acid) – gated or glutamate-gated chloride channels. Moxidectin stimulates the release of GABA and increases its binding to the postsynaptic receptors, and binds to the glutamate-gated chloride channels. The net effect is to open the chloride channels on the postsynaptic junction to allow the inflow of chloride ions and induce and irreversible resting state.
This result in flaccid paralysis and eventual death of parasites exposed to the drug.
Triclabendazole is a flukicide belonging to the benzimidazole group of anthelmintics. It is well established that benzimidazole anthelmintics selectively bind to ß-tubulin, thus causing the depolymerisation of microtubules and the subsequent disruption of microtubule – based processes in helminthes.
Moxidectin is distributed throughout the body tissues but due to its lipophilicity the highest drug concentrations are obtained in fat tissue. Moxidectin undergoes biotransformation by hydroxylation.
The majority of the oral dose of triclabendazole in rats, sheep, goats and rabbits is eliminated in faeces after 6-10 days, as unchanged drug or products of biliary excretion. Urinary excretion is minimal.
Indications of use
For the treatment of mixed nematode and fluke infections, caused by moxidectin and triclabendazole sensitive strains of:
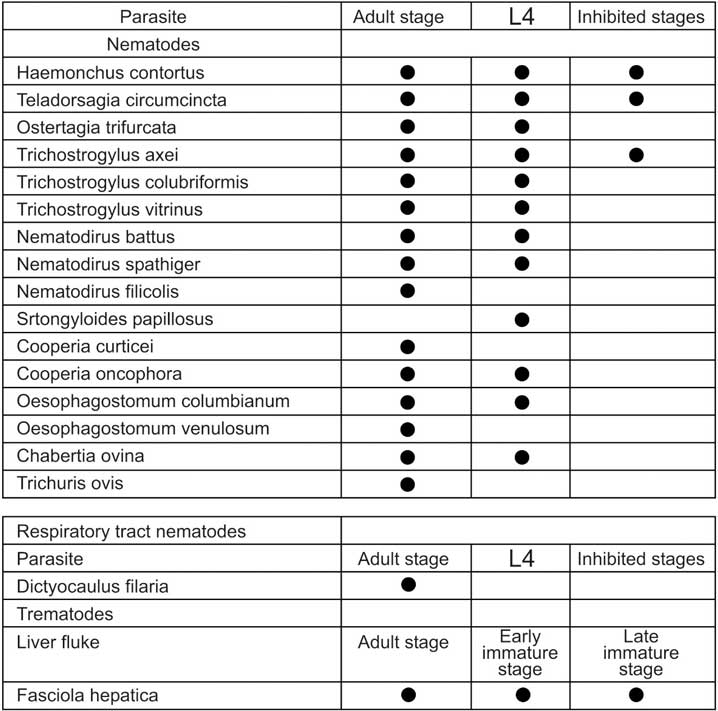
Dosage and route of administration
Should be given as a single oral drench of 1 ml/5 kg bodyweight, equivalent to 0.2 mg moxidectin/kg bodyweight and 10 mg triclabendazole/kg bodyweight, using any standard drenching equipment.
To ensure a correct dosage, body weight should be determined as accurately as possible; accuracy of the dosing device should be checked. If animals are to be treated collectively rather than individually, they should be grouped according to their body weight and dosed accordingly, in order to avoid under – or overdosing.
Contraindications:
Do not use in cases of hypersensitivity to the active substance(s) or to any of the excipient(s).
Adverse Reactions:
None known
Withdrawal Time
Meat: 31 days
Milk: not authorized for milks that consumed for use in human consumptions. This includes the dry periods.
Recommendations:
This product is safe for use in breeding animals.
Avoid direct contact with skin.
Do not smoke, drink or eat when suing this product.
Wear impermeable rubber gloves during use.
Wash hands after use.
This product should not be used for the treatment of single
infection. All animals in a group should be treated.
Suspected clinical cases of resistance to anthelmintics should be further investigated using appropriate tests (e.g. Fecal Egg Count Reduction Test).
Shelf-life after first opening the immediate package in.
All medication and this product should be kept out of the reach of children.
Storage conditions
Keep the product below 25 °C and avoid from direct sunlight.
Do not freeze.